Concept of GxP In Pharmaceuticals:
The concept of GxP requirements (GxP Compliance in pharma) was recognized by the US FDA (That is United States Food and Drug Administration) for a series of compliance-related activities mainly related to safety of pharmaceutical products.
- GxP is a set of rules and standard quality guidelines that aim to address the safety of the pharmaceutical product in an organized and comprehensive way while maintaining the quality of processes during every stage of pharmaceutical manufacturing, Quality control, storage, and distribution.
- While there is no solitary Authorities for GxP Compliance in pharma, guidelines and regulations, some of the common regulators include FDA in the US, TGA (The Therapeutic Goods Administration) in Australia, The Brazilian Health Regulatory Agency (Anvisa), and HCSC (Health Care Service Corporation) in Canada.
Brief GxP Compliance:
- GxP is a universal abbreviation for the “Good Practice” quality guidelines and regulations.
- The “x” stances for the various fields, including the pharmaceutical and food industries, for example, Good Documentation Practices (GDP).
- Sometimes the initialism is prefixed with a “c” or “C.” The letter “c” before it stands for “current.”
- For example, cGMP full name is “Current Good Manufacturing Practice (cGMP )”.
- A group of quality standards are usually referred to collectively as “GxP.”
- The goal of the GxP quality standards is to guarantee that a product is secure and serves its intended function. In regulated industries like food, medicines, medical devices, and cosmetics, GxP directs quality manufacturing.
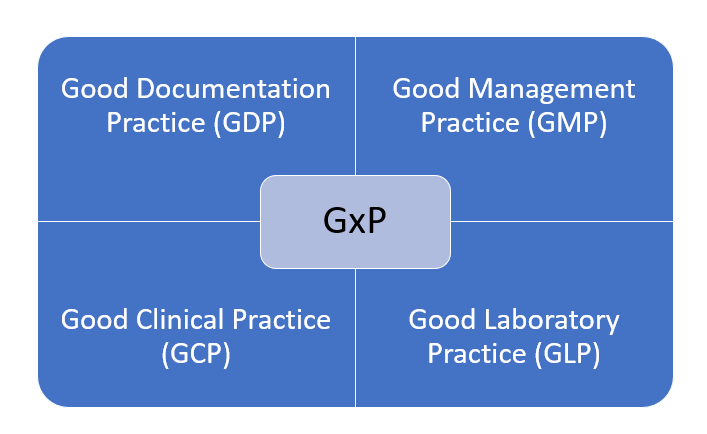
GxP stands
- In brief discussion GxP stands for ,
- G: Stands for good
- x: Variable
- P: Stands for practices
GDP and ALCOA Importance in GxP
- The Good Documentation Practices (GDP), which are anticipated to be ALCOA, are the most important parts of GxP:
- Attributable: Data must be connected to the individual who created the data. It clearly specifies who recorded the data or performed the activity, who wrote it and when.
- Legible: Documents are readable
- Contemporaneously Recorded: not dated in the past or the present, but rather when the task is finished
- Original: stands for a True Copy of documents
- Accurate: accurately reflecting the activity documented and Permanent,
- The products covered by the GxP compliance in pharma are anticipated to be
- Traceability: It is the ability to recreate the development history of a drug or medical device.
- Accountability: It is the skill to resolve who has contributed what to the development and when.
- Data Integrity (DI): Ensuring the reliability & consistency of e-data.
- 21 CFR 11 compliance
A Quality System must be created, implemented, documented, and maintained in accordance with GxPs.
Why is GxP important in Pharmaceuticals?
- Each business that manufactures products for the life sciences industry is impacted by GxP rules since they are worldwide in scope. Meeting the GxP standards is therefore crucial.
- Even though there are many GxPs, only a select few are crucial to the lifespan of any product.
GMP: Good Manufacturing Practices (GMP):
- Guideline on GMP refers to the recommendations made by regulatory bodies for the licensing and supervision of the production of goods like medicines, medical devices, active pharmaceutical ingredients (APIs), etc.
- Following these recommendations gives the agencies confidence in the products’ quality and shows that the makers have done everything within their power to ensure the products’ safety.
- The guidelines for good manufacturing practices, or GMPs, are set by organizations that control the manufacturing of medications. To reduce the hazards associated with any pharmaceutical manufacturing process, the rules establish minimum requirements that producers must adhere to it.
- The GMPs address the important factors to take into account when manufacturing drugs, such as: The scale and condition of the facilities are appropriate.
- The equipment is up to date and in good operating order. Processes are dependable and consistent.
GCP: Good Clinical Practices (GCP):
- Good Clinical Practices –GCP are global quality standards demonstrated by the International Conference on Harmonization (ICH) that state the clinical trial rules for the products that require testing on human subjects.
- The Guideline stipulates what constitutes a clinical study as well as the obligations of the officials participating.
- It makes sure that no tests on humans are carried out only for the sake of advancing medicine.
GLP: Good Laboratory Practices (GLP):
- These are the requirements established by the FDA for non-clinical laboratory testing and research done to evaluate the product’s safety and efficacy.
- GLPs are a set of guidelines that specify the framework for non-clinical studies and specify how they have to be carried out, assessed, reported, etc.
GDP: Good Distribution Practices (GDP):
- Good Distribution Practice (GDP) expresses the minimum condition that must be satisfied by a wholesale distributor to preserve the quality and integrity of the drugs across the supply chain.
- Good Distribution Practices -GDP is administered and regulated by global and national directions in the same way as Good Manufacturing Practices (GMP).
- The following are assured by GDP systems:
- Supply chain drugs are approved regulatory market, which are among the most stringent Medicines are processed in acceptable conditions and transported
- The inventory is rotated properly.
- Facility systems should permit employees rapidly to detect and repossess objects if needed Contamination of other goods is disallowed in all cases
- Pharma businesses must adhere to GxPs such as cGMP (Current Good Manufacturing Practices), GCP (Good Clinical Practice), GLP (Good Laboratory Practice), GPvP (Good Pharmacovigilance Practice), etc. in addition to GDP and GMP.
- In order to achieve successful compliance, firms must contact a committed and experienced Regulatory consulting team.
Who does GxP impact?
- GxP has an impact on regulated industries such as food, pharmaceuticals, medical devices, and cosmetics.
Why GxP Compliance in pharma important?
- GxP Compliance assures the security of goods and services.
- It enforces very particular and secure manufacturing processes and storage practices within regulated industries.
- GxP provides effective research standards for non-clinical laboratory investigations and guarantees the safety of clinical trials involving human beings.
- It has carefully organized the medicinal drug sector and is a benchmark that is acknowledged around the world.
- GxP refers to the unmistakable establishment of diverse contributions, in part or in whole, to a certain product or study and relies on exceptionally effective and efficient documentation.
- Every stage of the manufacturing process is recorded in order to trace the product’s lineage and confirm each intermediate step it underwent throughout production.

Examples of GxPs:
| Full Name of GxPs | Abbreviation or Short Form |
| Good Agricultural and Collection Practices, | GACP(s) |
| Good Agricultural Practice | GAP |
| Good Auditing Practice | GAP |
| Good Automated Laboratory Practice | GALP |
| Good Automated Manufacturing Practice | GAMP |
| Good Business Practice | GBP |
| Good Cell Culture Practice | GCCP |
| Good Clinical Data Management Practice | GCDMP |
| Good Clinical Laboratory Practice | GCLP |
| Good Clinical Practice | GCP |
| Good Documentation Practice | GDP GDocP (to distinguish from “good distribution practice”) |
| Good Distribution Practice | GDP |
| Good Engineering Practice | GEP |
| Good Financial Practice | GFP |
| Good Guidance Practice | GGP |
| Good Horticultural Practice | GHP |
| Good Hygiene Practice | GHP |
| Good Laboratory Practice | GLP |
| Good Management Practice | GMP |
| Good Manufacturing Practice | GMP |
| Good Microbiological Practice | GMiP |
| Good Participatory Practice | GPP |
| Good Pharmacovigilance Practice | GPvP or GVP |
| Good Pharmacy Practice | GPP |
| Good Policing Practice | GPP |
| Good Recruitment Practice | GRP |
| Good Research Practice | GRP |
| Good Safety Practice | GSP |
| Good Storage Practice | GSP |
| Good Tissue Practice | GTP |
References:
- European Medicines Agency (EMA)
- Food and Drug Administration (FDA)
- International Conference on Harmonization (ICH)
- Organisation for Economic Co-operation and Development (OECD)
Read More:
- Controlled Drug Delivery System
- pH Value Basic Principle of pH Meter
- pH Electrodes why are pH values mostly in a range of 0-14 ?
- Autoclave Principle
- GxP in Pharmaceuticals
- what is gxp in pharmaceutical industry
- Good Practice (GxP) in the Pharmaceutical Industry
- Climatic Zones for Stability Study
- Autoclave Sterilization Cycle Basic Parts of
- Autoclave
- Packaging Material used in Pharmaceuticals
- Testing of Glass Containers
- Primary Packaging
- Sterilization in Pharmaceuticals
- Autoclaving
- Difference between Class B and Class N Autoclaves Bowie Dick Test
- Vacuum leak test in Autoclaves
- Types of Autoclaves
- LAL test
- LAL test Procedure
