What is Limulus Amebocyte Lysate (LAL Test) ?
- Full form of LAL test is Limulus amebocyte lysate.
- LAL test is an aqueous extract of blood cells (amoebocytes) from the Atlantic horseshoe crab Limulus polyphemus.
- LAL reacts with bacterial endotoxin lipopolysaccharide (LPS), which is a membrane component of gram-negative bacteria.
- This reaction is the basis of the LAL test, which is widely used for the detection and quantification of bacterial endotoxins.
- In Asia, a similar Tachypleus amebocyte lysate (TAL) test based on the local horseshoe crabs Tachypleus gigas or Tachypleus tridentatus is occasionally used instead.
- The recombinant factor C (rFC) assay is a replacement of LAL/TAL based on a similar reaction.
History:
- The blood of the horseshoe crab will solidify (Semi-Solid Mass) into a semi-solid mass if gram-negative bacteria are present, according to a 1956 report by American medical researcher “Fred Bang”.
- It was later discovered that granules containing the clotting agent coagulogen, which is secreted outside the cell when bacterial endotoxin is encountered, are present in the animal’s blood cells, mobile cells referred to as amoebocytes.
- In the animal’s semi-closed circulatory system, the ensuing coagulation (gelling) is thought to contain bacterial infections.
- Multiple enzymes operate in a cascade to form the gel, as discovered through contemporary examination of the lysate.
- Modern Advanced analysis of the lysate has managed to understanding of this system of cascade, with multiple enzymes working in sequence to produce the gel.
- Limulus clotting factor C is the moment at which endotoxin-induced clotting starts
- LAL was authorized for use in the year 1977 by the U.S. Food and Drug Administration (FDA).
- Previously, a much slower and costlier test on rabbits had been used for same purpose.
- Horseshoe crabs are gathered, their pericardiums have blood extracted from them, and the crabs are then put back into the ocean.
- After being centrifuged to separate the blood cells from the serum, they are submerged in distilled water, where they swell and explode (“lyse”).
- This causes the cell’s internal compounds to be released as “lysate,” which is then cleaned up and dried using a freezer.
- A sample is combined with lysate and water to test for endotoxins; if coagulation happens, endotoxins are present.


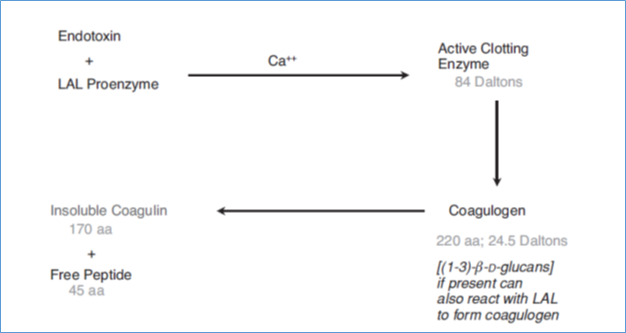
LAL Reaction Mechanism
- A proenzyme of LAL with a molecular weight of 150,000 is activated by endotoxin or a properly produced lipid-A derivative of endotoxin.
- Additionally, the presence of divalent metal cations like Calcium (Ca), Manganese (Mn), or Magnesium (Mg) is necessary for activation.
- It has been demonstrated that utilising LAL reagent with 50 mM magnesium can boost the sensitivity of the LAL assay for endotoxin detection by a factor of 10 to 30.
- After being activated, a lower molecular weight protein fraction (MW=19,000–25,000), also present in the LAL material, combines with the activated proenzyme. This protein fraction belongs to the serine protease class, which includes enzymes like thrombin, trypsin, and factor Xa.
- The proenzyme splits the lower molecular weight portion, known as coagulogen, into soluble and insoluble subunits. The lower molecular weight fraction, called coagulogen, is cleaved by the proenzyme into a soluble and insoluble subunit.
- Depending on how much of an insoluble coagulogen by-product forms, the insoluble component can take the shape of a solid clot, a precipitate, or a murky solution.
- As a result, in addition to endotoxin, three other components are needed for the coagulation response. The LAL reagent contains a clotting enzyme, clottable protein (coagulogen), and specific divalent cations.
- A schematic illustration of the LAL reaction mechanism is found as below in Figure.

Related: LAL Test Procedure
Reference:
- Ref. Book: Sterile Drug Products Formulation, Packaging, Manufacturing, and Quality by Michael J. Akers
