ICH Guideline: Q1A (R2) : Stability testing of new drug substances and products
ICH : International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
Brief About ICH Guideline: Q1A (R2) : Stability testing of new drug substances and products
- Application within the three regions of the EC, Japan, and the United States.
Purpose:
To check stability testing is to provide suggestions on how the quality of a drug substance or drug product differs with time under the effect of a variety of environmental factors such as temperature, humidity, & light, and to establish a re-test period for the drug substance or shelf life for the drug product and suggest storage conditions.
Four climatic zones, I-IV as per ICH Guideline
| ICH Stability Zones/ Zone | Type of Climate |
| Zone I | Temperate zone |
| Zone II | Mediterranean/subtropical zone |
| Zone III | Hot dry zone |
| Zone IVa | Hot humid/tropical zone |
| Zone IVb | Hot/higher humidity |
- This guideline addresses climatic zones I and II. covered
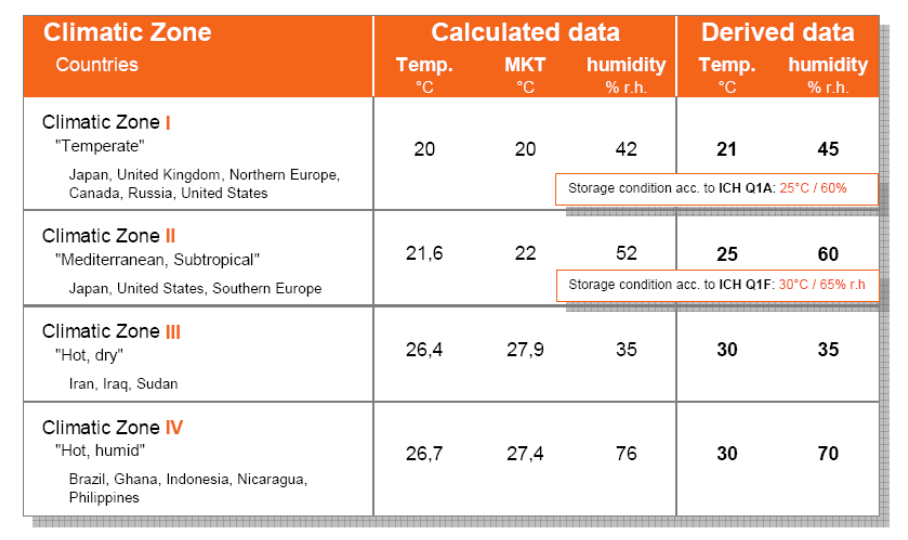

- General case
| Study | Storage condition | Min. time period performed/covered by data at submission |
| Long term* | 25°C ± 2°C/60% RH ± 5% RH or 30°C ± 2°C/65% RH ± 5% RH | 12 months |
| Intermediate** | 30°C ± 2°C/65% RH ± 5% RH | 6 months |
| Accelerated | 40°C ± 2°C/75% RH ± 5% RH | 6 months |
- Drug substances proposed for storage in a refrigerator
| Study | Storage condition | Min. time period performed/covered by data at submission |
| Long term* | 5°C ± 3°C | 12 months |
| Accelerated | 25°C ± 2°C/60% RH ± 5% RH | 6 months |
- Drug substances proposed for storage in a freezer
| Study | Storage condition | Min. time period performed/covered by data at submission |
| Long term* | – 20°C ± 5°C | 12 months |
- In the absence of an accelerated storage condition for drug substances (API) should be planned to store in a freezer, testing on a single batch at an elevated temperature (e.g., 5°C ± 3°C or 25°C ± 2°C) for a proper time period should be conducted to address the effect of short-term excursions outside the proposed label storage condition, e.g., during shipping or handling.
- Data from stability studies should be on at least three primary batches of the drug product.
- The primary batches should be of the same formulation and packaging system as proposed for marketing purposes.
Testing Frequency as per ICH Guideline
- Long term storage condition should be every 3 months over the 1st year, every 6 months over the 2nd year, and annually subsequently through the proposed shelf life.
Time Point: 0M, 3M, 6M, 9M, 12M, 24M, 36M
- Accelerated storage condition, a minimum of 3(three) time points, including the initial and final time points (e.g., 0M, 3M, and 6 months), from a 6-month study, is advisable.
Time Point: 0M, 3M, 6M.
- Intermediate storage condition is called for as a result of significant change at the accelerated storage condition, a min. of four-time points, including the initial and final time points (e.g., 0, 6, 9, 12 months), from a 12- month study is advisable.
Time Point: 0M, 6M, 9M, 12M.
- Pilot scale Batch: A batch of a drug substance or drug product mfg. by a procedure fully demonstrative of and pretending that to be applied to a full production scale batch. For solid oral dosage forms, a pilot-scale is generally, at a min., one-tenth that of a full production scale or 1 Lakh tablets or capsules, whichever is the larger.
Read More:
- Analytical Method Validation
- ICH Guidelines
- Type of HPLC Column
- Type of Capsules Advantages and Disadvantages of Capsules
- Gelatin
- Type of HPLC Detectors
- Difference between HPLC and UPLC
- What are the Differences between GC and HPLC?
- Data integrity
- Controlled Drug Delivery System
- pH Value
- Basic Principle of pH Meter
- pH Electrodes why are pH values mostly in a range of 0-14 ?
- Autoclave Principle
Reference:
- ICH Guideline
- If you have any Question, Click Here
- Contact US
