LAL Test Procedure:
- Even though the LAL test is very easy to perform, particularly when compared to the USP rabbit test, certain requirements must be followed. Limulus Amebocyte Lysate (LAL Test) consist of the following:
- It is necessary to properly clean and depyrogenate all materials that will come into touch with the LAL reagent or test sample.
- The reaction temperature must be in the range of 36◦C to 38◦C.
- The pH should be between 5–7 for reaction mixture.
- No more than an hour should pass before the response.
- There must be both positive and negative controls for every test.
- The basic process of the LAL test is the mixture of 0.1 mL test sample (TS) with 0.1 mL LAL reagent.
- The mixture is examined for the presence of a gel clot following an hour at 37°C of incubation.
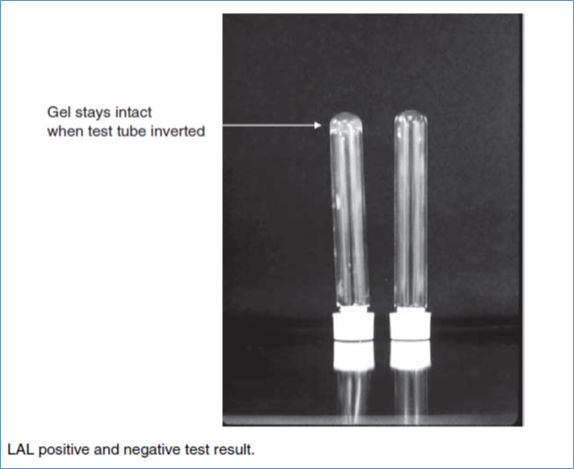
- If the gel clot remains intact following a slow inversion of the test tube holding the mixture, the LAL test is positive, confirming the presence of endotoxin. (Ref. figure).
- Standard: USP Reference Standard Endotoxin (RSE)

Manual Limulus Amebocyte Lysate Test Procedure:
- The majority of the time, four or more replicate samples are utilised for the test samples at each step of the dilution series.
- Unless otherwise stated in the specific monograph, the reaction mixture’s pH must range from 6.0 to 7.5.
- By adding sterile, endotoxin-free 0.1 N sodium hydroxide (NaOH), 0.1 N hydrochloric acid (HCL), or appropriate buffers, the pH can be changed.
- A test sample is added to test tubes that are typically 10 by 75 mm in size together with an aliquot of the reconstituted LAL reagent, typically 0.1 mL.
- Equal amounts of LAL reagent and endotoxin standard are added in other test tubes. Along with the test samples and endotoxin standards.
- Positive controls (LAL reagent samples with known endotoxin concentrations) and
- Negative controls (LAL reagent plus an equal volume of sterile, pyrogen-free solvent) are also performed.
- The test tube is gently spun (swirled) after combining the equal contents.
- The tube is positioned in a water bath that is kept at a constant temperature of 37°C. The optimal incubation period is 60 ± 2 min.
- The test tubes must never be moved while they are incubation to avoid accidentally disengaging any gel clots that may have formed.
- For the gel clot analysis, it is crucial to carefully remove the incubated test tubes.
- Instrumental analysis or direct visual observation can both be used to gauge the extent of gel formation.
- The test tube must first be carefully removed from the incubator before being gently turned 180 degrees and seen visually to determine whether a firm gel has formed.
- Conclusion of LAL Test:
- Positive Reaction: When a hard gel forms and maintains its integrity throughout and after the inversion process, that is a sign of a positive reaction.
- Negative result: It is defined by the lack of a gel or the production of a viscous gel that loses its integrity during the inversion process.
Ref. Book: Sterile Drug Products Formulation, Packaging, Manufacturing, and Quality by Michael J. Akers.
