What is Good Documentation Practice (GDP) and ALCOA++ ?
Good Documentation Practice (GDP): The procedure of preparing and handling of cGLP documents to maintain data integrity.
GxP: Acronym for the group of good practice guides governing the preclinical, clinical, manufacturing and post-market activities for biologics, medical devices, regulated pharmaceuticals etc., such as good laboratory practices (GLP), Good Clinical Practices (GCP), Good Manufacturing Practices (GMP) and Good Distribution Practices (FDP).
Documentation: A written or printed form which is used to furnish information or provide instructions. Any procedures, instructions, logs, records, raw data, manuals and policies associated with the development, testing, manufacturing, of medicinal product required to demonstrate compliance and any other applicable regulatory requirements.
Data integrity: Data integrity is defined as generating, transforming, maintaining and assuring the accuracy, completeness and consistency of data over its entire life cycle in compliance with applicable regulations.
Data: The information generated for quality evaluation and decision.
Metadata: Data that defines the attributes of other data. Most commonly these are data that describes the structure, data elements, inter-relationships and other characteristics of electronic records.
Raw Data: The actual information obtained from an observation, test, measurement or activity. This may contain computer or instrument printouts.
Records: Records include the raw data that provides evidence of various action taken to demonstrate compliance with the instructions/defined procedures.
Report: Document that creates the details about the conduct of particular exercises, projects or investigations, together with results, conclusions and recommendations.
Electronic Records: All original records and documentation, including data directly entered into computer through an interface, which are result of original observations and activities necessary for reconstruction and evaluation of raw data.
What does ALCOA and ALCOA++ stand for in Good Documentation Practice (GDP)?
What does alcoa stand for ? alcoa principles
ALCOA+ +: A commonly used ALCOA acronym (alcoa meaning) for “Attributable, Legible, Contemporaneous, Original and Accurate” which puts additional emphasis on the attributes being “Complete, Consistent, Enduring and Available”– qualities which are implicit in the basic ALCOA principles.
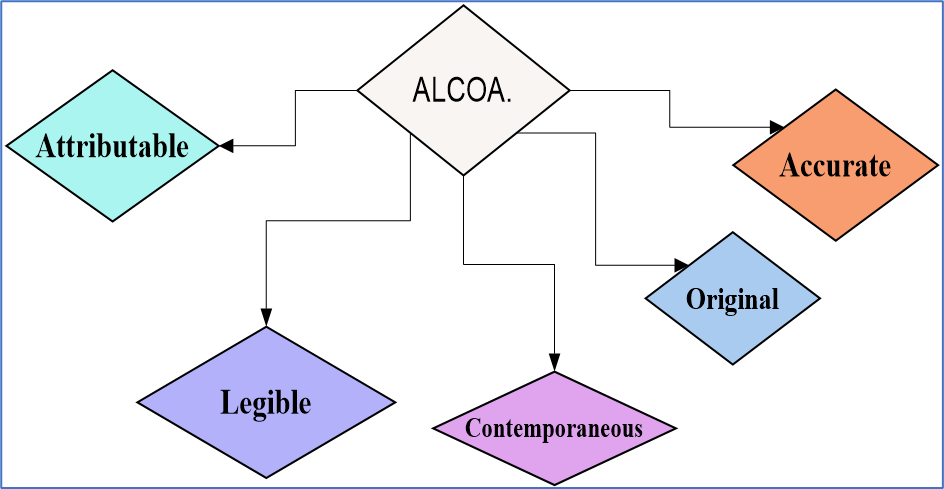

- Alcoa, an acronym for Attributable, Legible, Contemporaneous, Original, and Accurate, is a set of principles that guide the maintenance of data integrity. It has been widely adopted by industries that require complete, consistent, and enduring data, such as the pharmaceutical industry, and is highly valued by regulatory agencies like the Food and Drug Administration (FDA).
- Alcoa principles:
- The Alcoa principles were originally developed for paper-based data collection, but are also applicable to electronic records.
- The FDA has emphasized the importance of data integrity in the pharmaceutical industry, and has released guidance documents on how to maintain data integrity in electronic records.
- At its core, Alcoa is about ensuring that data is complete, consistent, and accurate.
- 1. Attributable: Data must be linked to the individual who created the data. It clearly indicates who recorded the data or performed the activity, who wrote it and when. The principles require that data be attributable, meaning that it can be traced back to its source. This is important in pharmaceutical manufacturing, where knowing the origin of data can be critical in identifying potential quality issues or deviations from good manufacturing practice.
- 2. Legible: Data is clear, concise and readable. The principles also require that data be legible, which means that it can be read and understood without difficulty. Changes to legible data must not hide or unclear the original record.In the pharmaceutical industry, legibility is crucial to ensure that information can be accurately transcribed and shared between different departments or organizations.
- 3. Contemporaneous meaning in ALCOA: Activity is documented at the time of the activity, Contemporaneous data recording is another key aspect of the Alcoa principles. This means that data should be recorded at the time it is generated, rather than being entered into a system at a later time. Time stamps and date and time information are important to ensure that data is recorded accurately and can be easily tracked over time.
- 4. Original: Data must be original record or certified copy. The originality of data is also important to maintain its integrity. This means that data should not be copied from other sources, and should be created and recorded independently. Additionally, data should be accurate and should reflect the actual results or observations.
- 5. Accurate: Data is correct through the system’s lifecycle and should have the same value and its correct meaning.
-
- Adherence to the Alcoa principles is crucial to comply with regulatory requirements in the pharmaceutical industry. For example, the FDA requires that electronic records be stored in a manner that ensures their accuracy and integrity, and that an audit trail is maintained to track any changes made to the records.
- The Alcoa principles can be applied to both paper-based and electronic records, but electronic records require additional considerations to ensure their integrity. For example, electronic records must be protected from unauthorized access or modification, and appropriate controls must be put in place to ensure that changes to the records are properly documented.
- The Alcoa principles are also relevant in the context of good manufacturing practice (GMP), which is a set of regulations and guidelines that ensure that pharmaceutical products are consistently produced and controlled according to established quality standards. Data integrity is a critical component of GMP, and the Alcoa principles are an essential tool for ensuring that data is accurate and reliable.
- In summary, the Alcoa principles are a set of guidelines that are essential to maintaining data integrity in the pharmaceutical industry. They are highly valued by regulatory agencies like the FDA, and are crucial for compliance with GMP regulations and guidelines. The principles can be applied to both paper-based and electronic records, but electronic records require additional considerations to ensure their integrity. Adherence to the Alcoa principles is critical for maintaining the quality and safety of pharmaceutical products, and is essential for ensuring that data is complete, consistent, and enduring
+ + (alcoa plus) elements include:
- Complete: All data including any repeat or reanalysis performed is considered complete data
- Consistent: Data is consistent when all elements of the analysis such as sequence of events follow on and are dated or time stamped in expected sequence
- Enduring: Data recorded in a permanent, maintainable form for the shelf life.
- Available: Data is available for review and audit or inspection over lifetime of record.
More:
- ALCOA GDP
- Alcohol Dose Dumping
- 21 CFR 11
- Female Infertility
- Inflammatory Bowel Disease (IBD)
- In-Vitro-Fertilization (IVF)
- Tablet Friability
- Quality Assurance
- Theoretical plate numbers (N) and Determination of “N” in Chromatography
- Preparation and Standardization of 0.1 N Hydrochloric Acid (0.1 N HCl)
- Difference Between Isocratic and Gradient Elution
- Calibration of HPLC
- Good Documentation Practice (GDP) & ALCOA++
- Gas Chromatography Columns
- Why is 70% the More Effective Concentration of Isopropyl Alcohol for Disinfection?
Reference:
