What is Friability Test of Tablet?
Friability is defined as the % weight loss of powder from the surface of the tablets due to a mechanical process and the test is performed to measure the weight loss during transportation or movement of Tablets.
It’s a crucial test that’s carried out throughout the tablet’s production to verify that it won’t break or chip during coating, packaging, or normal handling during distribution to the patient. It is an additional test for Uncoated / Compressed Tablets other than physical measurements like Hardness (Tablet Breaking Force).
Because some formulations, when compressed into particularly hard tablets, tend to ‘cap’ or lose their crown parts on attrition, measuring the hardness of a tablet is not a good predictor of tablet strength. Such tablets are prone to powdering, chipping, and fragmentation.
In the friability test, the tablets are prone to abrasion, allowing us to assess tablet strength under various force applications.
USP Standards for Friability Test Apparatus:
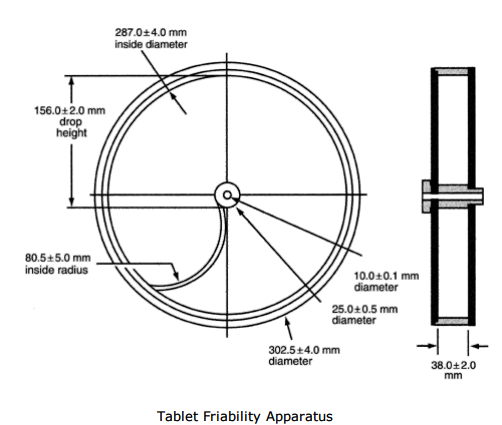

| Sr. No. | Title | Specification |
| 1 | MOC (Materials of Construction ) and Property of Drum | Transparent Synthetic Polymer with polished internal surfaces. Develops minimum Static charge. One side is removable. |
| 2 | Drum attachment | Horizontal to the axis of the Instrument |
| 3 | Internal Diameter | 283.0 to 291.0 mm (287.0 ± 4.0 mm) |
| 4 | Depth | 36.0 to 40.0 mm (38.0 ± 2.0 mm) |
| 5 | Outer Diameter of Central Ring | 24.5 to 25.5 mm (25.0 ± 0.5 mm) |
| 6 | Curved Projection with inside Radius (Extends from middle to outer wall of drum) | 75.5 – 85.5 mm (80.5 ± 5.0 mm) |
| 7 | Drum Revolution/Minute | 24 – 26 (25 ± 1) |
| 8 | Drop Height | 154.0 – 158.0 mm (156.0 ± 2.0 mm) |
| 9 | Total Revolution / Test | 100 Revolutions / 4 Min. |
| 10 | Function | At each turn, the Tablets roll or slide and fall onto the drum wall or onto each other |
Who is the responsible person to perform the test?
- Production officer/ executive
- IPQA officer
- Quality control officer
- F&D Scientist during development
What is the Friability test procedure?
- Collect an equal number of tablets from both sides of the chute and place them on a label. If the unit weight is equal to or less than 650 mg, use a tablet with a weight of 6.5 g or less.
- If tablets have a unit weight of more than 650 mg, then collect 10 (ten) Nos. tablets as a whole sample.
- Always check calibration status before performing the operation.
- Dedust all tablets and note down the weight performed on calibrated weight balance, put the required quantity of sample (Tablets) into the drum.
- Set all parameters (i.e rpm, Time) & start the test.
- After completion, dedust the whole tablet and weight again on calibrated weight balance.
- Calculate the friability as described in the calculation part.
- If the result falls out of the limits, reperform the test three (3) times and calculate the average of it.
- If the shape or size of an uncoated tablet is causing Tambling to be irregular, the friability drum can shift at 10º.
Precaution: Always do proper dedusting of tablets before and after the test to avoid an error.
Calculation:
Friability (%) = (W1 – W2)/ (W1) X 100
Where,
W1 = Weight of Tablets (Initial / Before Tumbling) &
W2 = Weight of Tablets (After Tumbling or friability)
What is the Acceptance Limit of Friability Test?
- Acceptance Limit of Friability (%) = Not More Than 1.0 %
Factors affecting friability of tablets :
- A weak tablet may be due to a many of factors, that could be poor tablet design, low moisture content in preparation, in-sufficient binder & over-lubrication.
Calibration of Friability Test Apparatus: Click Here
Read More:
- Blood Pressure:
- Alzheimer’s Disease:
- Alcohol Dose Dumping:
- Analytical-instruments-category:
- ICH
- Types of Autoclaves
- LAL test LAL test Procedure
- TLC & HPTLC
- What is friability of tablets
- GxP in Pharmaceuticals
- Types of chromatography
- CIP and SIP in Pharmaceuticals
- Positive control and negative control in Microbiology
- Types of biological indicators for sterilization Sterility Assurance Level (SAL)
- D value and Z values in Microbiology
- SOP on “Operation and Cleaning of Vertical Autoclave”
- Vacuum Leak Test, acceptance criteria and pharmaceutical applications
- Quality Assurance
- Analytical Development or Quality Control
- Formulation Development
- Microbiology
- Health Topic
Reference:
USP <1216>, EP<2.9.7>, and other pharmacopeias.
