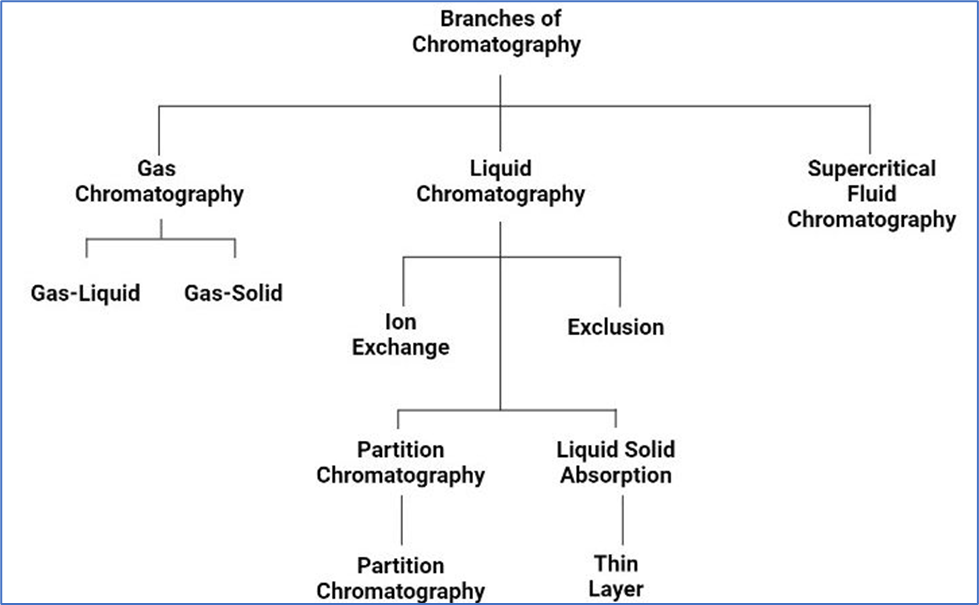
Classification of Chromatographic Techniques
- According to the type of equilibration process involved—which is determined by the kind of stationary phase—chromatographic procedures can be categorized. Various bases of equilibration are:
- Types of Chromatography:
- Adsorption Chromatography
- Partition Chromatography
- Ion Exchange Chromatography
- Size Dependent pore penetration (Size Exclusion Chromatography).
1. ADSORPTION CHROMATOGRAPHY
- The sample components are adsorbed on a solid surface known as the stationary phase.
- The mobile phase could be a liquid (liquid–solid chromatography) or as a gas (gas–solid chromatography); the components allocate between the two phases through a combination of sorption and desorption procedures.
- Thin-layer chromatography (TLC) is an example of adsorption chromatography. The stationary phase is planar, in the form of a solid maintained on an inert plate, and the mobile phase is in a liquid phase.
- The traditional type of liquid chromatography, referred known as liquid-solid or adsorption chromatography, was initially developed by “Tswett” around the turn of the 20th century.
- The usage of classic adsorption chromatography with solid stationary phases has decreased recently in favour of normal-phase chromatography due to the adaptability and easy accessibility of bonded stationary phases.
2. PARTITION CHROMATOGRAPHY
- In partition chromatography, the stationary phase is often a liquid supported on a solid or a network of molecules that practically operates as a liquid and is coupled to the solid support.
- Once more, the mobile phase can be a gas chromatography (gas-liquid chromatography, GLC) or a liquid (liquid-liquid partition chromatography).
- Liquid-liquid partition chromatography typically uses a non-polar mobile phase (e.g., hexane) and a polar stationary phase (for example, cyano groups bound to silica gel). Retention rises with increasing polarity when the system is exposed to analytes (dissolved in the mobile phase). This is known as Normal-phase chromatography (NPC).
- If a nonpolar stationary phase is used with a polar mobile phase (e.g. methanol, water, acetic acid), the retention of solutes decreases with increasing polarity. This mode of operation is called “Reversed-phase chromatography (RPC)” and is currently the most extensively used mode.
- To separate materials using chromatography, they must be soluble in the mobile phase. Compounds that are not water soluble are separated using normal-phase chromatography.
- It is more typical to utilize reversed-phase chromatography to separate water-soluble substances according to their varying levels of hydrophobicity.
- Partition chromatography can be subdivided into
- A. liquid-liquid and
- B. liquid bonded-phase chromatography.
- The way the stationary phase is retained on the packing’s support particles is what distinguishes the two.
- In liquid-liquid chromatography, the liquid is held in place by physical adsorption, whereas in bonded-phase chromatography, the liquid is attached by chemical bonding.
3. ION EXCHANGE CHROMATOGRAPHY
- Supports with ion exchange functionalities (abilities) are used as the stationary phase in ion exchange chromatography.
- Ion exchange equilibria are the basis of the separation mechanism. However, in the majority of ion exchange separations, particularly in anion exchange chromatography, hydrophobic interactions play a significant role.
- When it was demonstrated that anion or cation mixtures could be resolved on HPLC columns packed with anion-exchange or cation-exchange resins in the middle of the 1970s, ion chromatography as we know it today was first created.
- Ion chromatography is now performed using either a suppressor-based method or a single column.
- They differ in how the conductivity of the eluting electrolyte is prevented from interfering with the measurement of conductivities of analytes.
4. SIZE EXCLUSION CHROMATOGRAPHY OR GEL CHROMATOGRAPHY
- Size exclusion chromatography is also known as Gel Chromatography.
- In size exclusion chromatography, solvated molecules are divided based on their size by their ability to penetrate into porous pockets and passages in the stationary phase.
- Some types of chromatography, such as gas chromatography for gas-solid and gas-liquid chromatography, are regarded as distinct techniques.
- In every situation, subsequent equilibria dictate how much the analyte follows behind in the stationary phase and how much it follows the eluent (mobile phase).
- In column chromatography, the column can be filled & packed with small particles that act as the stationary phase (adsorption chromatography) or are coated with a thin layer of liquid phase (partition chromatography).
- In gas chromatography, a capillary column is an example in which a virtual liquid phase, frequently a polymer, is coated or bonded on the wall of the capillary tube.
Others,
5. AFFINITY CHROMATOGRAPHY
- A reagent called an “Affinity ligand” is covalently bonded to a solid support in affinity chromatography
- Affinity ligand examples are antibodies, enzyme inhibitors, or other molecules that reversibly and selectively bind to analyte molecules in the sample.
- This affinity chromatography technique is used for the purification of enzymes, nucleic acids, hormones, antibodies & specific proteins (dextran, polyacrylamide, cellulose etc).
- While free molecules leave the column, the specific molecules that forms a complex with the ligand is linked to the matrix and kept in the column.
- The engaged analytes eluted by changing the mobile phase conditions in this chromatography.
- Stationary phase is made of Solid, such as agarose, or a porous glass bead to which the affinity ligand is immobilized.
- The mobile phase in affinity chromatography has two different key requirements.
- It must support or facilitate the strong binding of the analyte molecules of sample to the Affinity Ligand.
- Second, the analyte-ligand association needs to be weakened or eliminated by the mobile phase after the unwanted species have been eliminated in order for the analyte to be eluted.
- Changes in pH or ionic strength are mainly used to change the elution situations throughout the two stages of the process.
- Affinity chromatography has the main advantage of extraordinary specificity.
6. CHIRAL CHROMATOGRAPHY
- A chiral chromatography is used for distinguished the chiral compound separation.
- A chiral auxiliary called a chiral derivatizing agent (CDA), also called a chiral resolving reagent, is used to transform a mixture of enantiomers into diastereomers so that the concentrations of each enantiomer in the mix may be determined.

Reference:
- Book:
- Fundamentals of Analytical Chemistry by Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
- Analytical Chemistry by Christian, Gary D., Purnendu K. (Sandy) Dasgupta, Kevin A. Schug
Read More:
- Bowie Dick Test
- Vacuum leak test in Autoclaves
- Types of Autoclaves
- LAL test
- LAL test Procedure
- TLC & HPTLC
- Types of Chromatography
- Type of HPLC Detectors
- Difference between HPLC and UPLC
- Paper Chromatography
- Types Chromatographic Techniques
- Calibration of HPLC
- Gas Chromatography Columns
