Preparation of 0.1 N Hydrochloric Acid (0.1 N HCl)
Preparation:
- Take a 100 mL of purified water or water in 1000 mL volumetric flask. Add 8.1774 (Approximately 8.2 mL of 37% Concentrated HCL) carefully. Add 700 mL water. Allow Solution to *cool down to Room Temperature. Make up the volume upto 1000 mL by water.
- * Because of Thermodynamic reaction volume may vary, so final volume shall be added after partial addition of volume.
Calculation:
- Molar mass for Hydrochloric Acid = 36.4611 g/mol
- HCL specific gravity is 1.189.
Now,
- Grams of compound required = (0.1 N) * (36.4611)* (1 Litre) = 3.6461 g
- Volume of concentrated acid required = (3.6461) / (0.375 x 1.189) = 8.1774 ml
- Therefore, 8.1774 ml of 37.5% concentrated HCL is required to prepare 0.1 M HCL.
Standardization :
1. Standardization of 0.1 N HCl (Hydrochloric Acid) with Sodium Carbonate (Na2CO3)
- Accurately weigh 0.5 to 1 g of Anhydrous Sodium Carbonate (Na2CO3) in suitable dish or crucible and keep dry at 250°C for approximately 4 hr.
- After completion of the activity allow to cool in a desiccator.
- Accurately weigh 0.22 g of dried Sodium Carbonate (Na2CO3) and transfer to 250 mL conical flask. Add 50 mL of water (H2O), mix well, and allow to dissolve the carbonate.
- Add 2 drops of 0.1% solution of methyl red in alcohol as an indicator.
- Titrate with HCl Solution filled in Burette accurately.
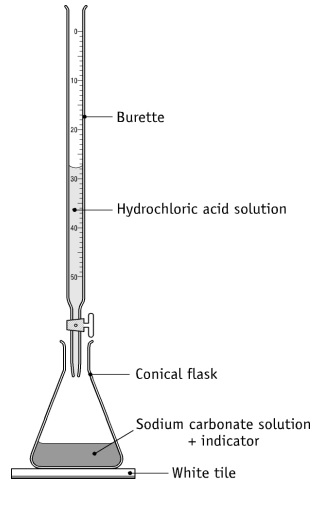

- Result: Clear solution on initial to yellow to peach pink on endpoint.
Calculation:
Na2CO3 + 2HCl = 2NaCl + H2O + CO2.
- The equivalent weight (wt.) of Na2CO3 is half its molecular weight (M). This means M/2 g of Na2CO3 is equivalent to 1000 ml of 1 N HCl.
- Calculate the molarity of HCl :
- m mol HCl = m mol Na2CO3
- (M * V) HCl = (M * V) Na2CO3 * 0.5
- (M * V burette) = (0.1 * 5) * 0.5
2. Standardization of 0.1 N HCl titrant with Tris(hydroxymethyl)-aminomethane (THAM or TRIS)
- Accurately weight 1.0 to 2.0 g of Tris(hydroxymethyl)-aminomethane (THAM or TRIS) ((HOCH2)3CNH2 ) in a suitable dish or crucible and keep for dry at 105°C for approximately 3 to 4 hr.
- After completion of activity allow to cool in desiccator.
- Accurately weight 0.50 g of dried THAM or TRIS and transfer to 250 mL conical flask. Add 50 mL of Water (H2O) which is ammonia and Carbon Dioxide free, mix well and allow to dissolve.
- Add 2 drops of Bromocresol Green as an indicator.
- Titrate with HCl Solution filled in Burette accurately.
- Each 121.14 mg tromethamine = 1 mL of 0.1 M Hydrochloride acid (HCl)
- Molarity of HCl = Wt. of TRIS/ 121.14 * Burette Reading of HCL
Read More:
Have any Query: Click Here
Contact Us: Click Here
