History of Paper chromatography :
- Paper chromatography was revealed by Synge and Martin in the year 1943.
What is Paper Chromatography?
- It is a chromatography separation technique that uses paper sheets or strips as the adsorbent material and the stationary phase through which a solution is forced to flow.
- It’s a cheap way to separate dissolved chemical compounds based on their differing migration speeds across paper sheets.
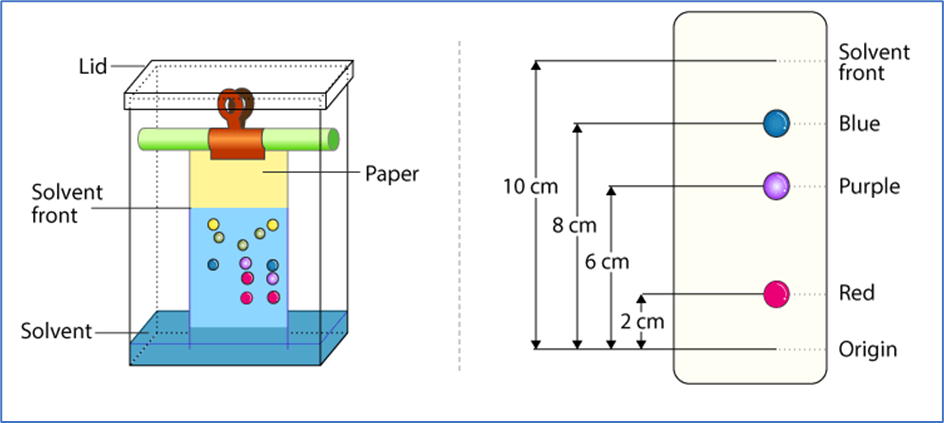

Principle and Technique of Paper Chromatography:
- The test solution or sample is applied as a spot near one corner of a sheet of filter paper in this approach.
- To establish a stationary liquid phase, the paper is first soaked with a suitable solvent to make it saturated. After that, one edge of the paper near the test location is soaked in another solvent in which the mixture’s components are soluble to varying degrees.
- The principle involved can be partition chromatography or adsorption chromatography.
- The solvent enters the paper by capillary action and carries the various components of the sample with it as it passes over the sample spot.
- The components travel at different speeds with the flowing solvent, depending on their solubilities in the stationary and flowing solvents.
- It has become usual practice for the separation of complex mixtures of amino acids, steroids, purines, peptides, carbohydrates, and a long list of simple organic compounds.
- Inorganic ions can also gladly be separated on paper chromatography than compare to thin-layer chromatography (TLC).
Procedure for Paper Chromatography:
- Selecting a suitable type of development: It is determined by the solvent’s, paper’s, and mixture’s complexity.
- Choosing a suitable filter paper: Filter paper is chosen based on the size of the pores and the quality of the sample.
- Prepare the sample: The dissolving of the sample in an appropriate solvent (inert with the substance under study) used to make the mobile phase is part of sample preparation.
- Spot the sample on the paper (Stationary Phase): Using a capillary tube, spot samples at the correct location on the paper.
- Chromatogram development: As a result of the capillary action of paper, the mobile phase travels over the sample on the paper (Stationary Phase).
- Paper drying and compound detection: The paper is dried with an air drier once the chromatogram has been generated. To identify the sample chromatogram spots, detecting solution can be sprayed on the chromatogram developed paper and dried.
Types of paper chromatography:
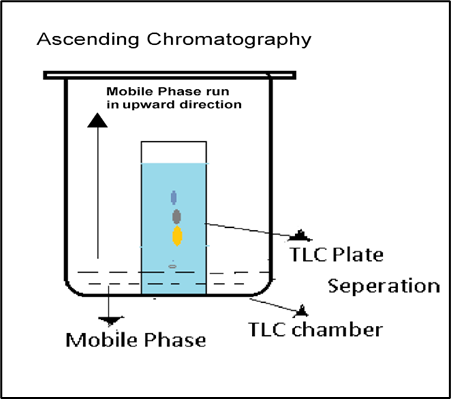
- Ascending Paper Chromatography: The process is named from the fact that the solvent travels upward.

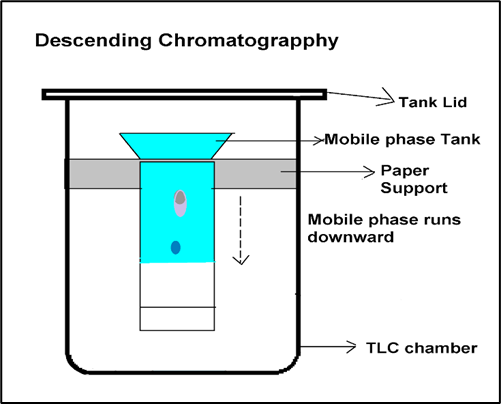
- Descending Paper Chromatography: Because of gravity attraction and capillary action, the flow of solvent in falling paper chromatography moves downwards.

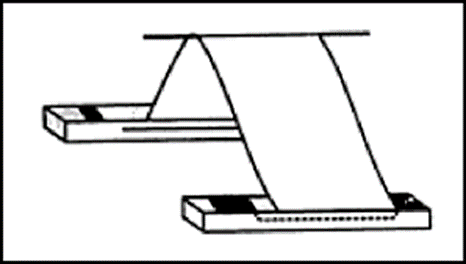
- Ascending -Descending Paper Chromatography: After a certain point, the solvent moves in two directions in this kind of chromatography. The solvent moves upwards on the paper that has been folded over a rod at first, then crosses the rod and continues its downward journey.

- Radial or Circular Paper Chromatography: The sample is sited in the circular filter paper’s centre. The filter paper is tied horizontally on a Petri plate containing the solvent once the spot has dried.
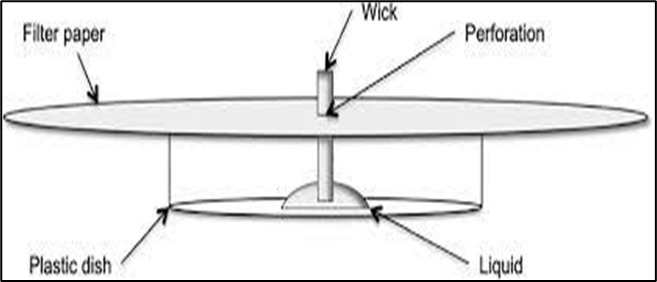

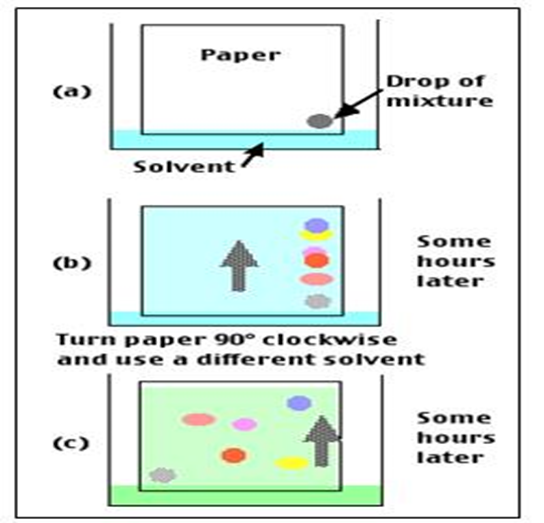
- Two Dimensional Paper Chromatography: Two-dimensional chromatography can be used to separate substances with similar rf values. The sample mixture is separated in the first solvent which is in nature of volatile. Than after it will be dried and paper should be turned 90° and separation carried out in another solvent. That will be useful in compound identification.

Paper Chromatography Applications:
- There are some useful applications of this chromatography. The following are some of the applications in various fields:
- To investigate the fermentation and ripening processes.
- To ensure that medications are pure.
- To examine cosmetics.
- Adulterants must be detected.
- To identify pollutants in beverages and meals.
- In biochemical laboratories, to examine reaction mixtures.
- To determine the presence of drugs and dopes in humans and animals.
Why water is not used in paper chromatography?
- It’s better to use a less polar solvent, like ethanol, because the non-polar molecules will go up the paper while the polar compounds will stay to the paper, separating them.
What are the limitations or drawback of Paper Chromatography?
- The following are some of the limitations :
- This chromatography cannot handle vast amounts of sample.
- In quantitative analysis, it is unsuccessful.
- Complex combinations are impossible to separate with this chromatography.
- HPLC or HPTLC are more accurate.
Reference:
- https://www.britannica.com/
- https://byjus.com/
- https://en.wikipedia.org/wiki/Paper_chromatography
- https://www.sciencedirect.com
Read More:
- Theoretical plate numbers (N) and Determination of “N” in Chromatography
- Preparation and Standardization of 0.1 N Hydrochloric Acid (0.1 N HCl)
- Difference Between Isocratic and Gradient Elution
- Calibration of HPLC
- Gas Chromatography Columns
- Why is 70% the More Effective Concentration of Isopropyl Alcohol for Disinfection?
- Difference between Incidence and Deviation
- Differential Scanning Calorimetry (DSC)
Topic on :
