- Biopharmaceutics Classification System (BCS): BCS is an experimental model that measures permeability and solubility under prescribed situations.
Type of BCS
| BCS Class | Class I | Class II | Class III | Class IV |
| Type | High permeability, High solubility | High permeability, Low solubility | Low permeability, High solubility | Low permeability, Low solubility |
| Bio-Availability | Well-absorbed and their absorption rate are usually higher than excretion. | The bioavailability of those products is limited by their solvation rate | The absorption is limited by the permeation rate but the drug is solvated very fast. | Those compounds have poor bioavailability. Generally, they are not well absorbed over the intestinal mucosa and a high changeability is expected. |
| Example | Metoprolol, Paracetamol | Glibenclamide, Bicalutamide, Ezetimibe, Aceclofenac | Cimetidine | Bifonazole |
The drugs are classified in BCS on the basis of solubility, permeability, and dissolution.
Solubility Determination:
The amount of substance that has passed into the solution when equilibrium is attained between the solution and excess (undissolved substance) at a given temperature and pressure is defined as the solubility of any substance.
When the highest dose strength of a drug substance or active pharmaceutical ingredient (API) is soluble in 250 mL or less of an aqueous medium over a specific pH range, it is considered highly soluble.
The volume evaluation of 250 mL is based on the typical volume of water consumed during dosage form oral administration, which is approximately 1 glassful, or 8 ounces of water. This boundary-value reflects (a light meal or repast) the minimum fluid volume expected in the stomach at the time of drug administration.
The pH solubility profile of the drug substance (API) is determined at 37°C in aqueous medium with pH in the range of 1 to 7.5 as per United States Food and Drug Administration (USFDA) guidelines,1.2 to 6.8 as per World Health Organization (WHO) guidelines and 1-8 as per European Medicines Academy (EMEA).
Summary:
- The boundaries of a solubility class are determined by the highest dose strength of an immediate release product. When the highest dose strength of a drug is soluble in 250 mL or less of aqueous media with a pH range of 1 to 7.5, it is considered highly soluble.
- The volume estimate of 250 mL is based on standard bioequivalence study protocols, which call for administering a drug product to fasting human volunteers with a glass of water.
Permeability Determination:
The methods that are normally used for the determination of permeability contain the following:
1. Human pharmacokinetic studies, including mass balance studies, absolute bioavailability (BA) studies, and intestinal permeability methods.
Unlabelled, stable isotopes, or radiolabeled drug substances are used in mass balance studies to determine the extent of drug absorption. In absolute BA studies, oral BA is measured and compared to intravenous BA as a control.
2. In vivo or in situ intestinal perfusion in an appropriate animal model
3. Methods for measuring permeability in vitro using excised intestinal tissues
4. Monolayers of suitable epithelial cells, such as Caco-2 or TC-7 cells
Summary:
- Permeability class boundaries are determined indirectly by the extent of a drug substance’s absorption in humans and directly by measuring rates of mass transfer across human intestinal membranes. Non-human systems accomplished of predicting drug absorption in humans can also be used (such as in-vitro culture methods). When the extent of absorption in humans is determined to be 90 percent or more of the administered dose based on a mass-balance determination or in comparison to an intravenous dose, a drug substance is considered highly permeable.
Dissolution
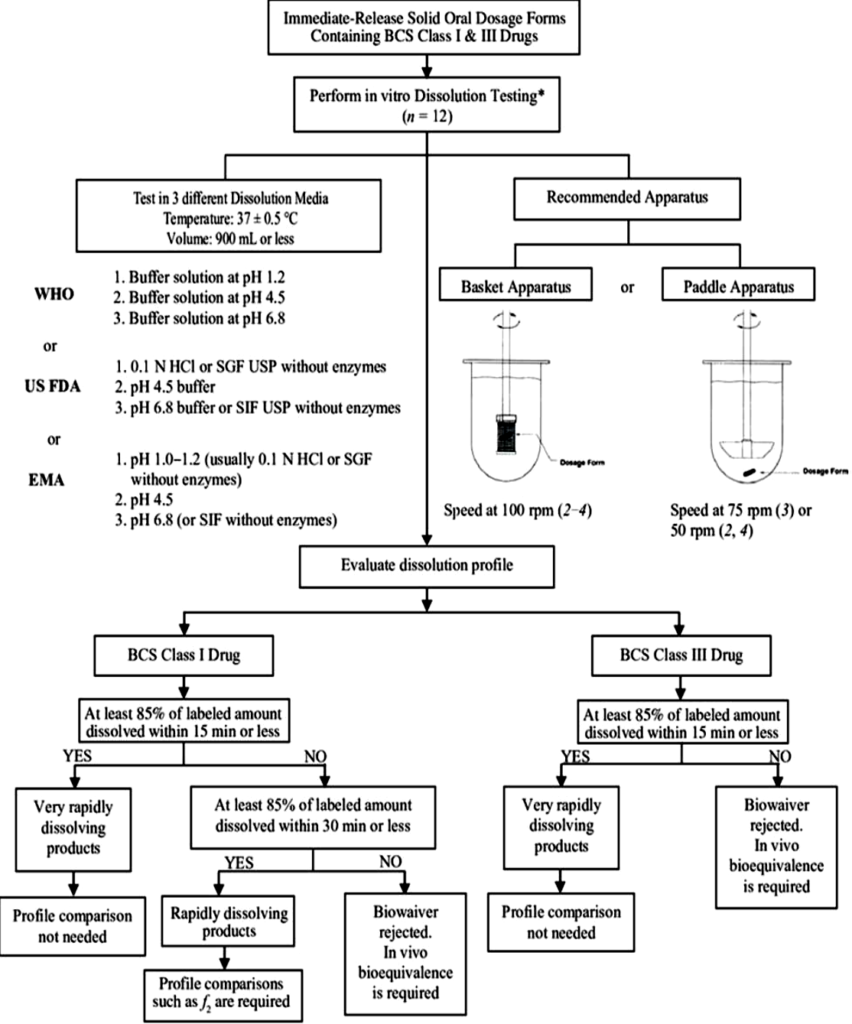
- As per USFDA BCS guidance an IR drug product is believed to be rapidly dissolving when NLT 85% of the labelled amount of the drug substance dissolves within 30 min., using USP apparatus I at 100 rpm (or Apparatus II at 50 rpm) in a volume of 900 mL or less in each medium:
1. HCl (0.1 N) or
2. simulated gastric fluid USP without enzymes;
3. Buffer (pH 4.5); and buffer (pH 6.8) or
4. simulated intestinal fluid (SGF ) USP without enzymes.
- As per WHO BCS guidance, a multisource product (pharmaceutically equivalent or pharmaceutically alternative products that may or may not be therapeutically equivalent) is believed to be very rapidly dissolving when NLT 85% of the labeled amount of the drug substance (API) dissolves in 15 min. using a paddle apparatus at 75 rpm or a basket apparatus at 100 rpm in a volume of 900 ml or less in each medium:
1. HCl solution (pH 1.2);
2. Acetate buffer (pH 4.5); and
3. Phosphate buffer (pH 6.8).
- As per EMEA BCS guidance drug products are believed to be very rapidly dissolving when more than 85% of the labelled amount is dissolved in 15 minutes, using USP Apparatus I at 100 rpm (or Apparatus II at 50 rpm) in a volume of 500 ml in each of the media:
1.HCl solution (0.1 N) or
2.Simulated gastric fluid (SGF) without enzymes;
3.Buffer (pH 4.5); and
4.Buffer (pH 6.8) or
5.Simulated intestinal fluid (SGF) without enzymes and
Similarity of dissolution profiles should be demonstrated.
Summary:
- For dissolution class boundaries, an immediate release product is believed to be rapidly dissolving when NLT 85% of the labelled amount of the drug substance dissolves within 15 min. by use of USP Dissolution Apparatus I at 100 RPM or Apparatus II at 50 RPM in a volume of 900 ml or less in the following media:
- M HCl or
- Simulated gastric fluid (SGF) or
- pH 4.5 buffer &
- pH 6.8 buffer or
- Simulated intestinal fluid (SIF).
Reference:
Read More :
- Difference Between Isocratic and Gradient Elution
- Calibration of HPLC
- Gas Chromatography Columns
- Why is 70% the More Effective Concentration of Isopropyl Alcohol for Disinfection?
- Difference between Incidence and Deviation
- Differential Scanning Calorimetry (DSC)
- Paper Chromatography
- Thin Layer Chromatography
- Difference Between Thin Layer and Paper Chromatography
- Type of Glass container used in Pharmaceuticals
- Fume Hood
- Type of HPLC Column
- Type of Capsules
- Advantages and Disadvantages of Capsules
- Gelatin
- Type of HPLC Detectors
- Difference between HPLC and UPLC
- What are the Differences between GC and HPLC?
- Data integrity
