Storage of pH Electrodes
- The recommended storage range for pH sensors (i.e. electrode pairs, combination electrodes, and differential types) is between 10°C and 30⁰C.
- The manufacturer’s instructions should be followed when installing the sensor’s protective and solution storage caps, which should be left in place.
- A 3.0 M to 3.5 M Potassium Chloride (KCl) solution works well as a storage solution.
- This solution gives the glass electrode a neutral to slightly acidic environment and prevents the imposition of a memory, similar to how incomplete discharge of Ni-Cad batteries can result in memory imposition.
- If KCl solution is not available, the following suitable alternatives are listed in order of preference:
- pH 4.0 buffer
- Distilled Water
- Tap water When these circumstances exist, the glass measuring
- Intermittently check to confirm that the storage solution has not evaporated from electrode.
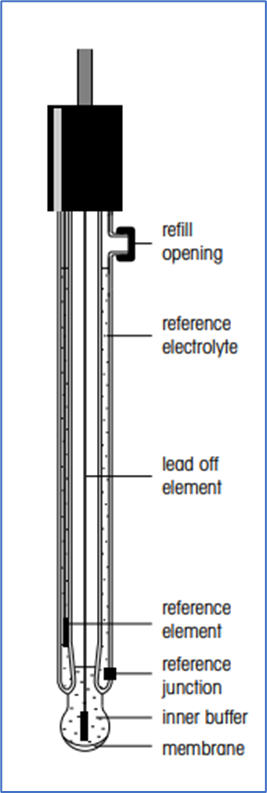

Related : Operation, Cleaning and Calibration of pH Meter
Preparation of 3 M Potassium Chloride (KCL) Solution:
- 22.365 g accurately weight 22.365 g dried KCL in the analytical balance. Add in the volumetric flask, and add 50 mL of Purified water to dissolve it. Add remaining 50 mL to make 100 ml 3 M KCL solution.
Calculations
- 100 ml* (3 mol/L) * (L/1000)* (74.55 g/mol) = 22.365 g KCL
Why are electrodes usually stored in a KCl solution?
- Potassium chloride (KCl) is the main source of chloride (Cl–) ions for the electrode.
- The advantage of using KCl for this purpose is that it is pH-neutral.
- In most cases, pH metres employ KCl solutions with concentrations ranging from 3 molar to saturated.
- The glass electrode and the reference electrode of the pH meter are submerged in the electrolytic solution, which is connected to the test solution (the pH of which is to be determined) through a porous ceramic membrane.
- The voltage is then shown on a voltmeter in terms of pH.
- Hence, we need such a solution in the electrolytic solution which would provide enough ions to complete the circuit and should also not contribute or disrupt or change the pH value of the test solution, since it is in a way, connected to it. Thus, we used KCl.
- Because the test solution is in some ways related to the electrolytic solution, electrode need a solution that will deliver enough ions to complete the circuit while also not interfering with, disturbing, or changing the pH of the test solution. So, it is better that we use KCl.
- Additionally KCl solution, is a good source of ions in the form of Cl−Cl− ions & it is neutral in nature resulting it does not participate or change the pH of the test solution.
Read More:
- Type of HPLC Column
- Type of Capsules
- Advantages and Disadvantages of Capsules
- Gelatin Type of HPLC Detectors
- Difference between HPLC and UPLC
- What are the Differences between GC and HPLC?
- Data integrity
- Controlled Drug Delivery System
- pH Value
- Quality Assurance Analytical Development or Quality Control
- Formulation Development
- Health Topic
