Different Capsule Dosage Forms
- Definition: Capsules are solid dosage forms wherein the drug substance is encased within either a hard or soft soluble shell.
- The capsule can be thought of as a “container” drug delivery system because it delivers a tasteless/odorless dose form without the requirement for a further coating procedure, as tablets do.
- Capsules are increasingly being used to deliver pharmaceutical and nutraceutical ingredients.
- These provide a number of advantages over tablets, which were previously the most extensively used dosage form.
- Empty capsules come in a variety of shapes, sizes, and materials, with each capsule holding a single dose of the active ingredient.
- As with tablets, certain other encapsulation excipients (include inert diluents, lubricants, and glidants, wetting agents, and disintegrants) antimicrobial preservatives, fillers, flavouring agents, sweeteners and coloring agents may be incorporated in the material that is loaded into a capsule.
- It could also be covered with a substance engineered to change the API’s release. The capsule’s outside surface can be printed with branding and dosing information.
- There are essentially two forms of capsule used for pharmaceutical and nutraceutical products – hard capsules and soft capsules.
- The shells are often made of gelatin, which is a widely formed animal-based product.
- HPMC (hydroxyprolymethyl cellulose) and Pullulan, which are now commercially available for both pharmaceutical and nutraceutical goods, have lately proven to be successful alternatives.

Types of Capsules
- Capsules are divided into the two types mainly,
- Hard-shelled capsules
- Soft-shelled capsules (Also known as “softgels”)
1. Hard Capsules (Two-piece gel encapsulation)
- Hard Capsules is also popular as two-piece capsules or dry-filled capsules
- Dry solid active substances are usually packaged in hard capsules. To make filling easier, the capsules are made in two halves: a body and a cap.
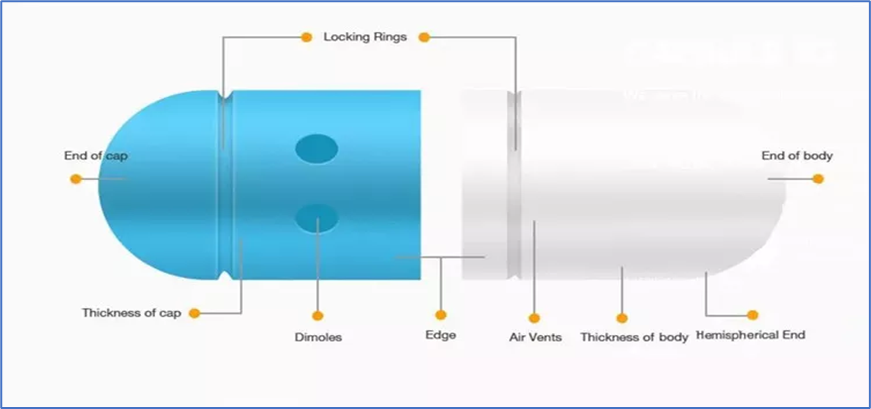
- In 1847, Londoner James Murdoch developed the two-piece telescopic gelatin capsule.
- Because a solution may gel and form a solid at a temperature just above room temperature (RT), gelatin has long been the material of choice for capsules.
- Traditional gelatin capsules are made by dipping finger-shaped pin forms into a hot gelatin solution at room temperature, removing them, and drying the resulting gelatin surface layer on the pins in a series of controlled air drying kilns.
- The capsules are peeled from the pins by bronze jaws and trimmed to length by stationary knives while being spun in chucks or collets once the film has dried.
- The cap and body portions are discharged from the machine after being cut to the proper length.
- The unlocked body and cap of each capsule are provided by the manufacturer, ready to be filled with the appropriate medication or nutraceutical contents.
- A pharmaceutical capsule can contain multiple types of drugs.
- Indeed, it is extremely typical for medications to be available in many forms, such as a tablet and a smaller capsule or powder. Both types of drugs can be packed into a larger capsule.
Empty capsules can be loaded with a variety of materials, including,
- Dry solids: Powders, pellets, granules or small tablets
- Semi-solids: suspensions or pastes
- Liquids: Non-aqueous liquids such as alcohols or oils
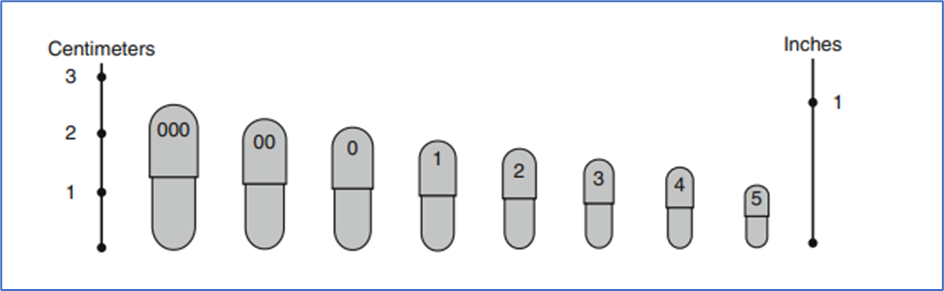
- Capsule Volumes and Estimated Fill Weights
| Size | Volume | Calculated fill weight (g) at powder density of 0.8 g/cm3 |
| 000 | 1.37 | 1.096 |
| 00 | 0.95 | 0.760 |
| 0 | 0.68 | 0.544 |
| 1 | 0.50 | 0.400 |
| 2 | 0.37 | 0.296 |
| 3 | 0.30 | 0.240 |
| 4 | 0.21 | 0.168 |
| 5 | 0.13 | 0.104 |
2. Soft Gelatin Capsule
- Soft gelatin capsules, also known as soft gels, are thicker than hard gelatin capsules and require specialized equipment, such as a rotating capsule machine or a dosator machine that creates capsules using the drop formation concept.
- A rotary capsule machine can produce capsules and insert fills in a continuous cycle. The average hourly output rate is between 25000 and 30000 capsules.
- A plasticizer, such as glycerine, sorbitol, or ethylene glycol, is commonly 20-30% by weight in soft capsule gelatin.
- The amount and type of plasticizer used to influence the end product’s hardness, as well as its dissolution and disintegration properties, as well as its physical and chemical stability.
- Soft gelatin capsule composition:
- The plasticizer’s composition was chosen to prevent any interaction or migration between the fill and the soft gel shell.
- To prevent bacterial growth on the gelatin, a preservative such as beta-naphthol might be used.
- Sweetening agents, as well as colouring agents and opacifiers, may be used.
- The thickness of the gelatin is determined by the item to be contained within the capsule, as well as the ambient temperature and humidity.
- In order to ensure optimal processing during gel preparation and soft gel encapsulation, soft gels typically comprise 30-40% water of the wet formulation.
- Following the filling of the capsule, the gelatin is gradually dried until the final capsule contains between 6% and 13% water by weight.
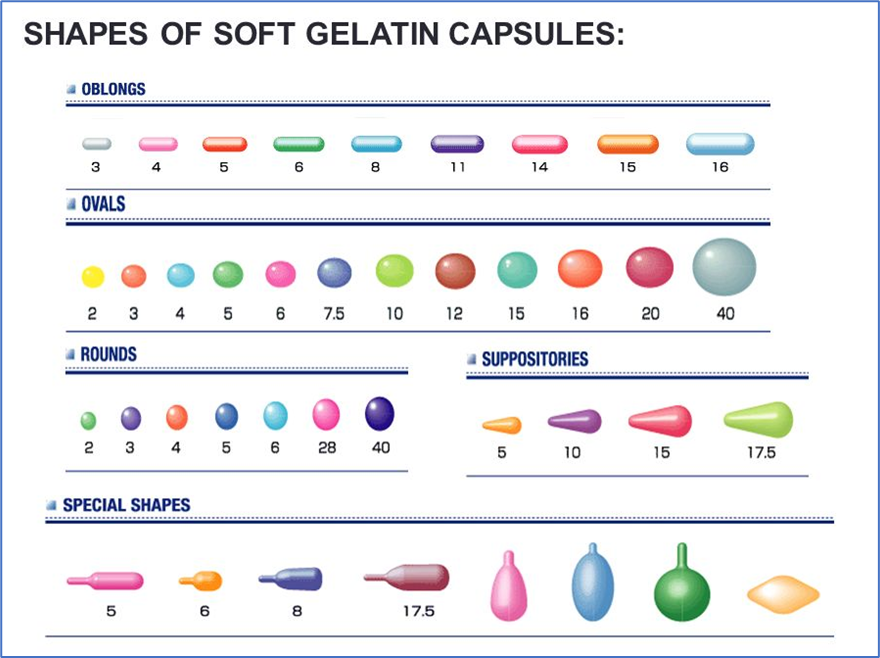
- Soft gel capsules are available in a variety of shapes and sizes e.g.,
- Cylindrical – 0.15 to 25 ml
- Ovoid – 0.05 to 7 ml
- Pear-shapeded – 0.3 to 5 ml
- Spherical – 0.05 to 5 ml
- Tubes – 0.3 to 5 ml
Soft gel capsules can be filled with three different types of materials::
- Pure or Neat substances (Example: Cod liver oil)
- Solutions:
- Active materials dissolved in a carrier such an oil (Like ,soybean),
- Polyethylene glycol (PEG)
- A Solvent that does not damage the gelatine shell (e.g., dimethyl isosorbined and diethylene glycol).
- Suspension or paste: Before viscosity and filling become an issue, materials with up to 30% solids can be tolerated.
- A minor amount of water or alcohol (up to 10% v/v) may be added to the fill if required to support the solubility of ingredients.
- Glycerin can also be added to retard any migration of plasticizer out of the gelatine shell into the fill.
- Maximum 10% by weight of polyvinylpyrrolidone may also be added in mixture with polyethylene glycol to increase stability of the fill.
3. Modified Release Capsule
- Chemical modifications can be made to both hard and soft gel capsules to change the active ingredient’s release (s).
- If the medicine is water-soluble and in a hard capsule, the excipients should be hydrophilic and neutral, whereas excipients that slow the release of water-soluble drugs should be hydrophilic and neutral.
- Rapid capsule release can also be achieved by piercing the outer film with small holes or adding a little amount of citric acid and sodium bicarbonate to aid in the opening of the capsule via carbon dioxide (CO2) evolution.
- To improve water penetration and expedite dissolving, a minute amount of sodium lauryl sulphate (up to 1%) can be added to the gel of a soft capsule.
4. Enteric Capsule
- Enteric capsules are a type of modified release capsule that can come in either a hard or a soft form.
- The encapsulating substance is made to withstand stomach acid until it reaches the intestinal fluid, where it breaks down and releases the active components at a higher pH.
- Cellulose acetate phthalate, as well as waxes and fatty acids and/or their esters, have been used as coatings for enteric capsules.
- These work by being soluble in alkali but insoluble in acid.
- Rather than being a function of pH alone, several contemporary coating materials have been discovered to degrade over time when exposed to gastrointestinal fluids.
- Such coatings need a lot of skill and extra equipment to apply, and they should probably only be used for drugs that are not in critical nature.
Related: Advantages and Disadvantages of Capsules
References
- Allen L. and Ansel H. (2014). Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems. Philadelphia: Lipincott Williams and Wilkins.
- Aulton, M. and Taylor, K. (2013). Aulton’s Pharmaceutics Book- The Design and Manufacture of Medicines, (4th ed.). Edinburgh: Churchill Livingstone.
- Modern Pharmaceutics, Fourth Edition, Revised and Expanded edited by Gilbert S.Banker, University of Iowa, Iowa City, Iowa.,Christopher T.Rhodes, University of Rhode Island Kingston, Rhode Island
Read More:
- Splitability Test-or-Breakability Test
- Difference-between-suspension-and-emulsion
- Attenuated Total Reflectance (ATR)
- Differential Scanning Calorimetry (DSC)
- Paper Chromatography
- Thin Layer Chromatography
- Difference Between Thin Layer and Paper Chromatography
- Type of Glass container used in Pharmaceuticals
- Fume Hood
- Type of HPLC Column
