About Titration Molarity or Normality :
What is a Titration?
Titration: It is a process of chemical analysis in which the qty. of some constituent of a sample is determined by adding to the measured sample an accurately known quantity of another substance with which the desired constituent reacts in a definite, known proportion.
It’s also known as “volumetric analysis” and “titrimetry.”
Type of Titration :
- Acid-base titration
- Redox titration
- Gas phase titration
- Complexometric titration
- Zeta potential titration
- Assay
- Back titration
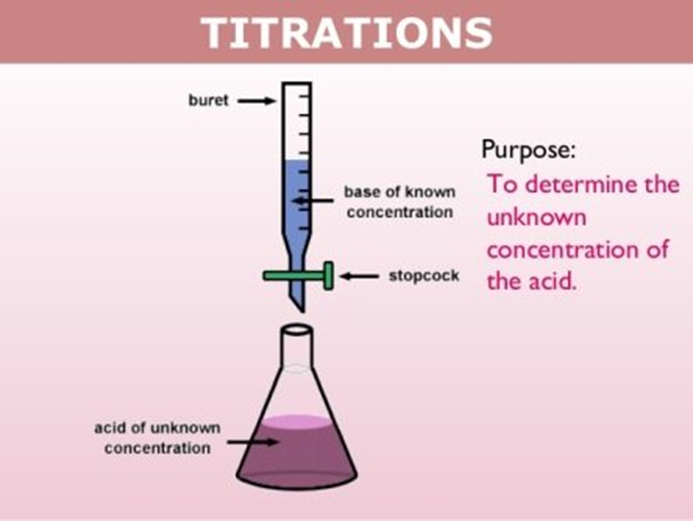

How to measure the endpoint of a titration?
1. Indicator:
In response to a chemical change, a substance changes color. The colour of an acid–base indicator (such as phenolphthalein) changes with the pH variation. At the start of the titration, a 1-2 drop of indicator solution is introduced in the solution; the endpoint is reached when the colour changes.
Type of Indicators:
| Indicator | Acidic side Color | Range of color change (pH) | Basic side Color |
| Methyl violet | Yellow | 0.0–1.6 | Violet |
| Bromophenol blue | Yellow | 3.0–4.6 | Blue |
| Methyl orange | Red | 3.1–4.4 | Yellow |
| Methyl red | Red | 4.4–6.3 | Yellow |
| Litmus | Red | 5.0-8.0 | Blue |
| Bromothymol blue | Yellow | 6.0–7.6 | Blue |
| Phenolphthalein | Colorless | 8.3–10.0 | Pink |
| Alizarin yellow | Yellow | 10.1–12.0 | Red |
2. pH meter
3. Conductivity
4. Color change
5. Precipitation
6. Isothermal titration calorimeter
7. Thermometric titrimetry
8. Spectroscopy
9.Amperometry
What are normality and molarity?
- Normality (N) is defined as the number of a gram (g) or mole equivalents of solute present in 1.0 litre of a solution.
- Normality = Number of gram equivalents × [volume of solution in litres]-1
- Molar Concentration or Molarity (M) is defined as the number of moles of solute present in a definite amount of liters of the solution, that is, moles per liters of a solution.
- Molarity (M) = No. of moles of solute × [volume of the solution in litres]-1
Differences Between Normality and Molarity
| Normality | Molarity |
| Equivalent concentration is another name for it. | Also known as molar concentration. |
| It is defined as the number of gram (g) equivalent per 1.0 litre of solution. | It is defined as the number of moles (mol.) per 1.0 litre of solution. |
| It’s used to calculate the gramme equivalent in relation to the solution’s total volume. | It is used in measuring the ratio between the number of moles in the total volume of the solution. |
| Units : N or eq L-1 | Unit: M or Moles L-1 |
Difference between Molarity and Molality:
| Point | Molarity (M) | Molality (m) |
| Measure of | Concentration | Concentration |
| Definition | The moles of a solute per solution (in liters) | The moles of a solute per solvent (in Kilograms) |
| Units | M | m |
| Equation | M = moles solute / liters solution | m = moles solute / kg solvent |
| Ratio of moles to: | Volume (in liters) | Mass (in kilograms) |
Specific gravity: The specific gravity is the ratio between the density of an object or desired substance, and a reference standard substance.
RD = ρ Substance ÷ ρ Reference
Read More:
0.1 N HCl Preparation and Standardization
Have any Query: Click Here
Contact Us: Click Here
