Classification of Climatic Zones for Stability Study
- The quantity of stability testing must be decreased in order to sufficiently reduce the variety of testing situations.
- When they established four distinct long-term test conditions that matched with the climatic circumstances of the target markets, which were divided into simply four separate climatic zones, Paul Schumacher and Wolfgang Grimm accomplished this in the year 1972 and 1986, respectively.
- As pharmaceutical items were being developed, this idea became the norm.
- The World Health Organization (WHO) put out the idea of dividing Climatic Zone IV into two distinct zones, IVA and IVB, in the year 2005.
- Four climatic zones, I-IV
| ICH Stability Zones/ Zone | Type of Climate | Limit (Mean Annual Temp. / Mean Annual water Vapour Pressure) | Testing conditions [ ◦C / % RH] |
| Zone I | Temperate zone | ≤15°C/ ≤11hPa * | 21 / 45 |
| Zone II | Mediterranean/subtropical zone | ˃ 15° to 22°C / ˃ 11 to 18 hPa | 25 / 60 |
| Zone III | Hot dry zone | ˃ 22°C /≤15 hPa | 30 / 35 |
| Zone IVa | Hot humid/tropical zone | ˃ 22°C / ˃ 15 to 27 hPa | 30◦C / 65% RH |
| Zone IVb | Hot/higher humidity | ˃ 22°C / ˃ 27 hPa | 30◦C / 75% RH |
*≤hPa- Hectopascal
Long term conditions for stability data :
| WHO and ICH guidelines Long term testing conditions (Temp. /Humidity) | Testing conditions | Climatic Zones | Long term Stability testing which could be realized in order to cover all climatic zones |
| 21°C/ 45% RH | Long term | Zone I | 25°C/ 60% RH |
| 25°C/ 60% RH | Long term | Zone I and II | 25°C/ 60% RH |
| 30°C/ 35% RH | Long term | Zone III | 30°C/ 65% RH |
| 30°C/ 65% RH | Intermediate Long term | Zone I and II Zone IV a | 30°C/ 65% RH |
| 30°C/ 75% RH | Long term | Zone IV b | 30°C/ 75% RH |
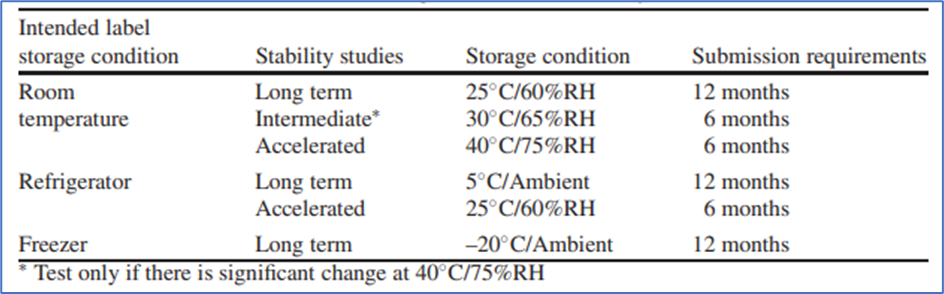
ICH storage conditions of stability studies

Classification of Countries According to Climate Zones
| Region | Zone I and II countries | Zone III and IV countries |
| Europe | All countries | NA |
| America | Argentina, Bolivia, Chile, Canada, Mexico, Peru, Uruguay, USA | Barbados, Belize, Brazil, Costa Rica, Dominican Republic, Ecuador, El Salvador, Guatemala, Guyana, Haita, Honduras, Jamaica, Columbia, Cuba, Nicaragua, Dutch Antilles, Panama, Paraguay, Puerto Rico, Venezuela. These nations are all grouped under CZ IV. |
| Asia | Afghanistan, Armenia, Azerbaijan, China, Georgia, Iran, Israel, Japan, Kazakstan, Kirghizia, Korea, Lebanon, Nepal, Syria, Tadzhikistan, Turkey, Turkmenia, Uzbekistan | Bahrain, Bangladesh, Hong Kong, India, Indonesia, Iraq(III), Jordan (III), Kampuchea, Qatar, Kuwait, Laos, Malaysia, Maldives Islands, Myanmar, Oman, Pakistan, Philippines, Saudi Arabia, Singapore, Sri Lanka, Taiwan, Thailand, United Arab Emirates, Vietnam, Yemen |
| Africa | Egypt, Algeria, Tunesia, Libya, Morocco, Namibia, Ruanda, South Africa, Tunesia, Zambia, Zimbabwe. | Angola, Ethiopia, Benin, Botswana (III), Burkino Faso, Burundi, Djibouti, Ivory Coast, Gabon, Gambia, Ghana, Guinea, Cameroon, Kenya, Longo, Liberia, Madagascar, Malawi, Mali, Mauritania, Mozambique, Niger, Nigeria, Senegal, Sierra Leone, Somalia, Sudan, Tanzania, Togo, Chad (III), Uganda, Zaire, Central African Republic. |
| Australian/ oceanic | Australia, New Zealand. | Figi Society Islands, Marshall Islands, New Caledonia, Papua-New Guinea, Samoa, Tonga. |
Realated: Q1A (R2) : Stability testing of new drug substances and products, Q2(R1): Validation of Analytical Procedures
Mean Kinetic Temperature (MKT)
- By proposing the Mean Kinetic Temperature (MKT) idea in the 1990s, Wolfgang Grimm made a significant advancement in the establishment of appropriate stability testing conditions based on sound research.
Definition of MKT:
- In assessing the effects of heat on pharmaceutical products, the MKT takes the reaction rate constants into consideration.
- The following is a reasonable definition of the MKT: MKT is the temperature that corresponds to how a certain temperature-time distribution affects the kinetics of chemical reactions.
- The MKT enables estimation of how temperature changes affect a substance’s chemical deterioration in a specific product.
Definitions of significant changes of data stored at accelerated conditions
- For Drug Substances (API):
- Significant change is defined as failure to meet the specification
- For Drug Products:
- A 5.0% potency change from the initial assay value.
- Any specified degradant beyond its acceptance criteria
- Does not meet acceptance criteria for appearance and physical properties (e.g., delivery per actuation, color, caking ,phase separation, re-suspendability, hardness); and as appropriate to the product type.
- The pH value above its acceptance criteria
- Dissolution exceeding the acceptance criteria for 12 dosage units.
Reference:
- International Conference of Drug Regulatory Authorities (ICDRA Acronym)
- International Federation of Pharmaceutical Manufacturers and Associations (IFPMA Acronym)
- Handbook of Stability Testing in Pharmaceutical Development written by Kim Huynh-Ba.
- ICH
- WHO
